Research and Development in the Pharmaceutical Industry
A Complex System Delivering Breakthroughs that Change Patients’ Lives

Research and development (R&D) is the foundation for discovering innovative medicines and vaccines that change patients’ lives. Pfizer’s R&D program is designed to develop safe and effective breakthroughs for patients across the globe.
Generally, the R&D process includes basic and translational research, in addition to medicines and vaccine development:
- Basic research: Investigates the biological underpinnings of disease and potential therapeutic targets. Performed by academic research centres, government agencies, and industry, often in close collaboration with one another.
- Translational research: Connects new knowledge about a process or compound to a practical medical application or therapy. Typically performed by biopharmaceutical companies.
- Medicine and vaccine development: Investigates the safety and efficacy of new medicines and vaccines through clinical trials. Typically performed by biopharmaceutical companies.

Drug R&D Is a Complex, Resource-Intensive Endeavor
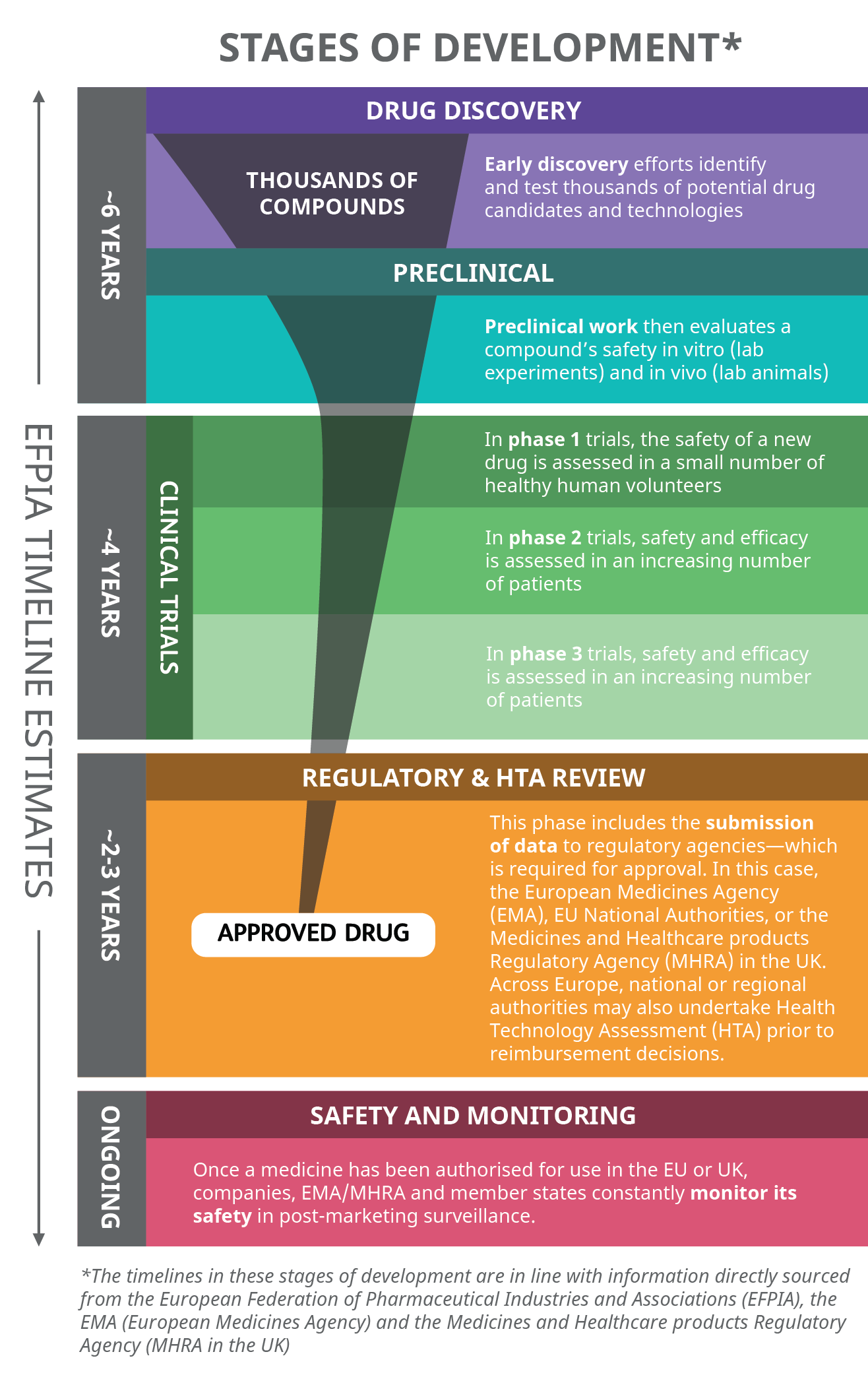
The development of a potential new medicine, medical product, or vaccine starts with drug discovery, in which thousands of compounds may be screened and/or assessed. The European Federation of Pharmaceutical Industries and Associations (EFPIA) estimates that on average, only 1-2 out of every 10,000 substances that are synthesised in laboratories will successfully become an actual medicine.1 After the initial screening phase of drug discovery, preclinical research in the form of laboratory experiments is carried out. If all preclinical criteria are met (e.g., safety requirements) and regulatory approval to conduct clinical trials is obtained, the extensive clinical trial process with human volunteers and patients begins. New medicines and vaccines are typically evaluated in many thousands of patients before receiving regulatory agency approval.2 As some population groups have historically been underrepresented, ensuring racial and ethnic diversity in clinical trials is critical.3
According to the European Medicines Agency (EMA), around 4,000 clinical trials are authorised across the European Economic Area (EEA) each year, with approximately 61% sponsored by the pharmaceutical industry.4 Before new medicines and vaccines are available to patients in Europe, they must undergo regulatory evaluation to confirm safety, quality, and efficacy prior to gaining marketing authorisation, and national or regional review and decisions on market access.
Not all potential medicines reach this point - a recent estimate puts the annual success rate across all therapeutic areas and clinical development stages (from phase 1 onwards) at an average of 13.1% (between 5-24.6%) for the years 2010-21, with the lowest figure (5%) in 2021, something that could reflect increased scientific risk and challenges in product development due to COVID-19.5 For those that are successful, EFPIA estimates it can take 12-13 years before a medicinal product reaches the European market 1—this period accounts for the years between the first synthesis of the new active substance and when it is made available for patients.
For those products that make it through phase 3 trials, a submission for marketing authorisation is made to the relevant regulatory authority. In the UK, this is the Medicines and Healthcare products Regulatory Agency (MHRA). In the EU/EEA, pharmaceutical companies can make a central application to the EMA in order to obtain marketing authorisation for all member states (this is mandatory in certain cases), or apply to the various national competent authorities.6, 7 Although the process of drug development and evaluation is similar across all EU member states and the UK, there are differences in regional and national Health Technology Assessment (HTA) and reimbursement processes, and wide variations in the mean time from marketing authorisation to patient access across Europe exist, from as little as four months to as long as 2.5 years. 6, 8
Many of these timelines were successfully accelerated during the COVID-19 pandemic, with multiple vaccines and therapeutics receiving conditional marketing authorisation in 2020/21.9, 10 Across multiple COVID-19 vaccines, pivotal trial timelines were reduced by 70% compared to a pre-COVID-19 benchmark5.This required huge mobilisation of resources across industry, academia and governments/regulators, alongside new approaches to R&D whilst retaining high quality and safety standards, such as running multiple processes in parallel rather than in sequence11
Importantly, this was only possible due to a long history of investment in R&D by industry, governments, and others, facilitated by incentives that support innovation across the R&D ecosystem. A major focus of today’s R&D ecosystem is understanding what can be learnt from this experience and embedded elsewhere to continue to deliver important breakthroughs to patients.
Overview of the R&D Process 12
Collaborating for R&D Success
Partnerships are central to R&D, and Pfizer welcomes opportunities to work with research partners in academia, government, and the private sector.
- Pfizer partnered with BioNTech to develop a vaccine for COVID-19, and then with the UK Vaccine Taskforce and European Commission to supply millions of doses.13,14
- The Innovative Medicines Initiative has been the world’s biggest public-private partnership in health research, with over 180 projects. Pfizer participated in over 40% of these across Europe15, and has recently led several new public-private projects including for rare diseases (Screen4Care), advanced therapies (ARDAT), new target discovery (REsolution) and Oncology (OPTIMA).16, 17, 18, 19
- Pfizer collaborated with AbbVie, Biogen Inc., and the Broad Institute of MIT and Harvard to enable access to the world’s largest browsable resource linking rare protein-coding genetic variants to human health and disease. This gives access to results from analyses of whole exome sequencing data from 300,000 UK Biobank research participants; the UK Biobank data were generated as part of the UK Biobank Exome Sequencing Consortium, formed in 2018, with several other industry partners.20
- Pfizer will invest over €520 million to support manufacturing and research in France. This includes aims to strengthen biomedical research through R&D investments in pre-clinical research collaborations, venture funding for French biotechs, and measures to facilitate clinical trial access for patients. 21
- Pfizer created the Innovative Target Exploration Network, an early stage partnering model that enables collaborations with select academic institutions to identify research projects that could have significant potential for future drug discovery.22
The Cost of Drug Discovery
The R&D process is increasingly costly, and because only a small percentage of drug candidates make it to approval, failure is the most common outcome.
- The estimated cost of researching and developing a new chemical or biological entity was estimated at €1.9 billion ($2.6 billion in 2013 dollars) in 2014.1
- The research-based pharmaceutical industry alone invested an estimated €37.8 billion in R&D in Europe (including UK) in 2019, and €39 billion in 20201. This is far higher than Eurostat’s estimates of total public spend on medical and health R&D, estimated at €10.3 billion in 2019 (EU28 inc. UK) and €9.1 billion in 2020 (EU27 only).23
12-13 years is how long it takes, on average, for a new medicine to be discovered and made available to patients in Europe.1
Our Commitment to Improving Health Through R&D
Pfizer is committed to investing in R&D as part of our mission to find breakthroughs that change patients’ lives. We are dedicated to ensuring the safety of all patients and volunteers who take part in our clinical trials, and we uphold the highest ethical standards in all our research initiatives. Understanding that some groups have historically been underrepresented, we are committed to ensuring racial and ethnic diversity in clinical trials. We are active supporters of finding new ways of discovering and developing these medicines and vaccines and accelerating patient access to these beneficial products. We welcome opportunities to partner with the public and private sectors to improve the R&D process. More information on Pfizer’s policies for clinical trials and our commitment to ethics is available at https://www.pfizer.com/science/clinical-trials.
Notes
1 EFPIA The Pharmaceutical Industry in Figures Key Data 2021: https://www.efpia.eu/media/602709/the-pharmaceutical-industry-in-figures-2021.pdf
2 FDA Drug Development Process: https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process
3 FDA Racial and Ethnic Minorities in Clinical trials: https://www.fda.gov/consumers/minority-health-and-health-equity/racial-and-ethnic-minorities-clinical-trials
4 European Medicines Agency: https://www.ema.europa.eu/en/human-regulatory/research-development/clinical-trials-human-medicines#:~:text=In%20the%20EEA%2C%20approximately%204%2C000%20clinical%20trials%20are%20authorised%20each%20year
5 IQVIA Global Trends in R&D in 2021: https://www.iqvia.com/Insights/The-IQVIA-Institute/Reports/Global-Trends-in-R-and-D-2022?utm_campaign=2022_R%26DTrends2022_Institute_TC&utm_medium=email&utm_source=Eloqua
6 "Drug development: the journey of a medicine from lab to shelf” (Marketing) https://pharmaceutical-journal.com/article/feature/drug-development-the-journey-of-a-medicine-from-lab-to-shelf
7 European Medicines Agency: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/evaluation-medicines-step-step
8 European Federation of Pharmaceutical Industries and Associations (EFPIA) Patients W.A.I.T. Indicator 2020 Survey: https://www.efpia.eu/media/602652/efpia-patient-wait-indicator-final-250521.pdf
9 Ball P. The lightning-fast quest for COVID vaccines - and what it means for other diseases. Nature. 2021 Jan;589(7840):16-18. doi: 10.1038/d41586-020-03626-1. PMID: 33340018
10 European Medicines Agency: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#authorised-covid-19-vaccines-section
11 https://www-nature-com.eu1.proxy.openathens.net/articles/d41573-020-00149-2
12 EFPIA The pharma industry – R&D with a focus on R&D process https://www.efpia.eu/publications/data-center/the-pharma-industry-in-figures-rd/rd-process/
13 “Pfizer and BioNTech to Provide European Union More Than 200 Million Additional Doses of COMIRNATY® to Help Meet Continued Need for Vaccine Supply ”: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-european-union-more-200-million
14 European Commission, “Coronavirus: Commission signs a third contract with BioNTech-Pfizer for an additional 1.8 billion doses”: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_2548
15 Pfizer EU research and innovation policy: https://www.pfizereupolicy.eu/article/eu-research-and-innovation-policy
16 Screen4Care: https://www.screen4care.eu/
17 ARDAT: https://ardat.org/
18 REsolution: https://re-solute.eu/resolution
19 OPTIMA: https://www.optima-oncology.eu/
20 “Collaboration between AbbVie, Biogen and Pfizer creates world's largest browsable resource linking rare protein-coding genetic variants to human health and disease”: https://www.pfizer.com/sites/default/files/partnering/recent_partnership/Abbvie_and_Biogen.pdf
21 “Pfizer announces €520 million investment in France to fight Covid-19”: https://www.france24.com/en/europe/20220117-pfizer-announces-%E2%82%AC520-million-investment-in-france-to-fight-covid-19
22 “Pfizer Establishes New Partnering Model for Early-Stage Academic Research”: https://www.pfizer.com/news/press-release/press-release-detail/pfizer_establishes_new_partnering_model_for_early_stage_academic_research