The European Innovation Ecosystem
The European pharmaceutical industry is a driving force for the European economy delivering a world-class environment for clinical research and pharmaceutical production. It is positioned as the second-largest pharmaceutical market globally, accounting for 22% of all pharmaceutical sales.

These factors underline the importance of fostering a nurturing environment that will maximise the pharmaceutical industry’s ability to harness scientific advances, address Europe’s health challenges, and continue to generate high-quality jobs and economic output for the European economy.
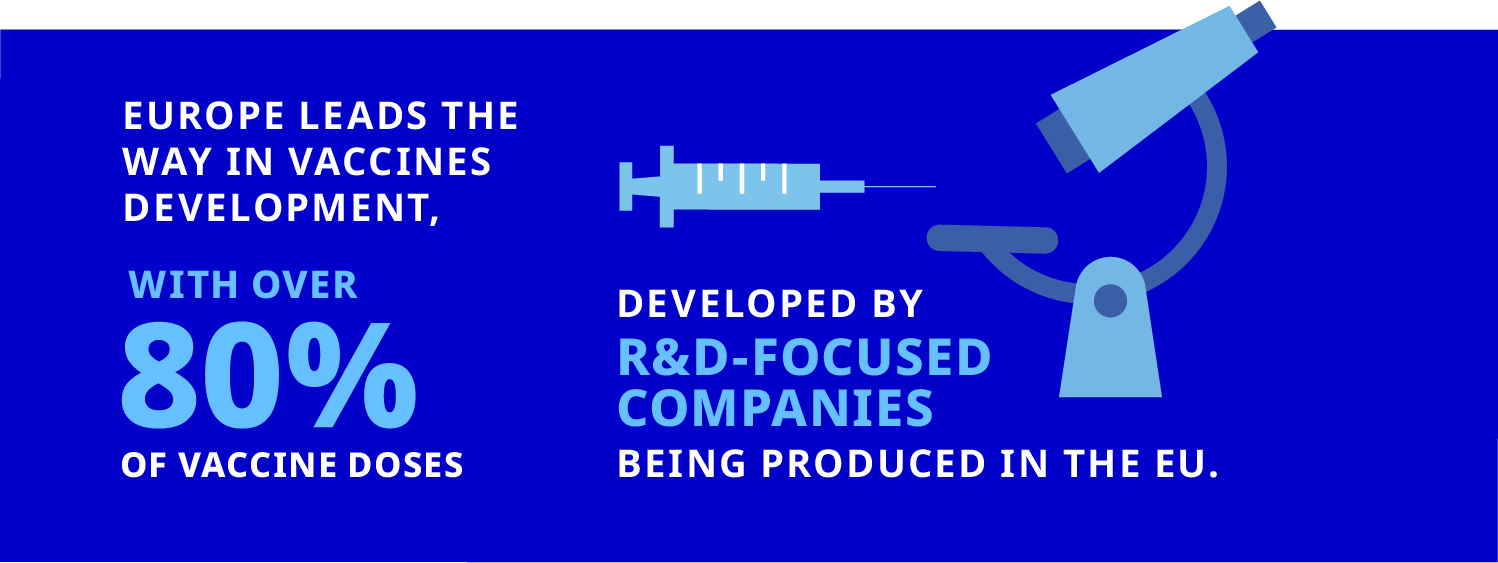
The Response to COVID-19
COVID-19 shone a very bright spotlight on the pharmaceutical sector and its ability to mobilise and respond to the threat that it posed.
The capacity of the pharmaceutical sector to respond to the challenges of the pandemic was the result of decades of investments in developing the science, technologies, and skilled people that power R&D, manufacturing, and commercialisation of biopharmaceutical and medical products. However, the geographic variability in “capacity to respond,” coupled with the differing speed and effectiveness of national responses, suggests that there are valuable lessons to be learned.
An integrated life sciences ecosystem approach builds sector-wide resilience in order to respond to future events on the scale of the COVID-19 pandemic. For more insights on the key factors which allowed the life sciences sector to respond effectively to these extraordinary circumstances, we invite you to read this report by TEConomy Partners.
Recommendation
At Pfizer, we believe that the EU must implement an interconnected set of pro-patient and pro-innovation policies to maintain a competitive life sciences environment that will allow the European pharmaceutical sector to flourish and continue to innovate.
European Innovation Policy Pillars
We propose that a healthy European Innovation Ecosystem needs to be built on an interconnected and inter-dependent set of building blocks. We have identified nine key pillars, which can deliver a future-proof approach for pharmaceutical innovation across Europe.
- Investing in a Robust Research and Development Infrastructure will allow innovators to discover, develop, and deliver therapies and vaccines for European citizens, and in doing so contribute to a strong European economy.
- Upholding Strong Intellectual Property Protections is vital to the development of innovative technologies and supports a robust, knowledge-based economy in Europe.
- Investing in People, as the European innovation ecosystem needs a diverse pool of highly skilled and technically trained workers to deliver both now and in the future.
- Harnessing the Power of Data and Digital Technology to accelerate innovation and support the ecosystem’s resiliency.
- Contributing to a Sustainable Green Economy to protect the environment, resources and communities in which we live and operate.
- Adapting the Regulatory System to reflect the new opportunities science offers to patients: this is necessary to deliver next-generation, safe, and high-quality vaccines and treatments to meet the health challenges of tomorrow.
- Improving and Accelerating Patient Access to medicines, addressing affordability concerns while ensuring the sustainability of health systems.
- Strengthening Supply Chain Resilience to meet patient needs, ensuring continuity of response in times of crisis and shortage.
- Championing Open and Ambitious Trade Policies, including advocating for a functional multilateral rules-based system that enables free and fair trade.
Enacting policies across these key pillars will help bolster the European innovation ecosystem and ensure that the pharmaceutical industry remains agile in responding to large-scale threats while continuing to innovate and transform the lives of European patients.
In delivering on this objective, we will continue to strengthen the resilience of the European economy and ensure Europe maintains its position as a global innovation powerhouse.